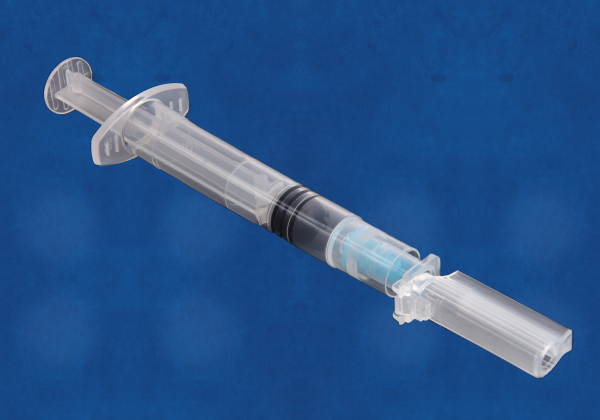
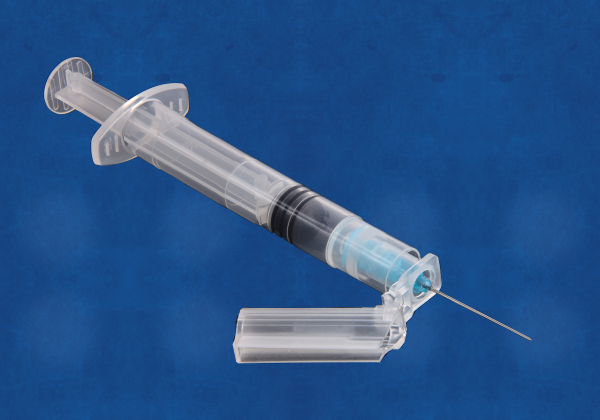
The K4 Safety Needle Cap device is a unique, high-quality, anti-needle stick device that exceeds functional standards, and meets global standards. Its novel design offers low costs of production, as well as unique product differentiation and excellent marketing opportunities in a crowded sector. K4 meets the highest standards (OSHA, USA) and ISO and complies with UNICEF and WHO guidelines.
Star is offering an opportunity for non-exclusive licensing for manufacture and distribution.
Product features:
- Universal K4 fits onto all three major syringe types – luer slip, luer lock and needle lock and capable of accommodating nearly all needle lengths and gauges.
- Unique 'transport position' K4 allows syringes to be filled in one location, then made safe temporarily while transported to where injection is administered.
- Rotation K4 cap can rotate around the needle hub to facilitate administration of low angled injections such as Mantoux technique for intra-dermal delivery and for needle bevel orientation when administering I.V. injections.
- Safe disposal After injection K4 is positioned to cover needle, then locked for full needle protection prior to safe disposal
- Replacement K4 is a 'like-for-better' replacement for the standard needle cap found on nearly all hypodermic syringes. Competitor products RETAIN the standard needle cap and either add additional components or require more complex syringe designs.
- Super low cost K4's unique design results in materials costs which are 50% less than most comparator products, as well as significantly lower tooling and assembly machinery costs.
WHO Policy
Safety is at the heart of the new global WHO policy, advocating the rational and safe use of injectables.
WHO policy states: “Millions of people could be protected from infections acquired through unsafe injections if all healthcare programmes switched to syringes that cannot be used more than once.”
This product is part of the solution, fully complying with the new regulations in a cost effective way. Click the button below to read the full WHO Policy.
About us
Star is a medical device licensing company that transfers its AD Syringe and Needle Safety technology to manufacturers around the world.
StarTek, a division of Star Syringe, was set up to provide our licensee base with a dedicated engineering resource for all ‘K’ products, and is a small group of highly experienced medical device engineers who have jointly invested over 90 years in the medical devices industry.
This range of expertise covers design, quality management, production, training, including turn-key solutions for the production of medical disposable AD syringes. The StarTek team members have built more than 30 factories for syringes, needles and associated products worldwide.
Whether technology transfer for our ‘K’ products, including full documentation, to a green field site with full turn-key factory solutions including training, we have the expertise to get the job done.

Rupert Lywood
CO-FOUNDER AND CHAIRMAN
Rupert has spent 25 years founding and building businesses in the medical, energy, information, finance, and media sectors – including co-founding and chairing a multi-billion Euro trans-European energy business, sold in 2007. He remains active in all these sectors and is also currently focused on literacy programs in the Developed and Developing World.

Norbert Rudolf
TECHNICAL MANAGER
Norbert joined Star in 2008, working within StarTek overseeing all aspects of Quality Assurance of K1 medical devices to ISO standards.
He now leads K1 and K4 engineering and consultancy for licensees, project planning up to implementation of a medical device factory.
His experience spans 30 years within the medical device sector.

David Wallace
MANAGING DIRECTOR
David is a chartered accountant and joined Star in 2015. He has a diverse background including project management and funding, tech transfer and was formerly head of Wildvision, the commercial arm of the BBC’s Natural History Unit.